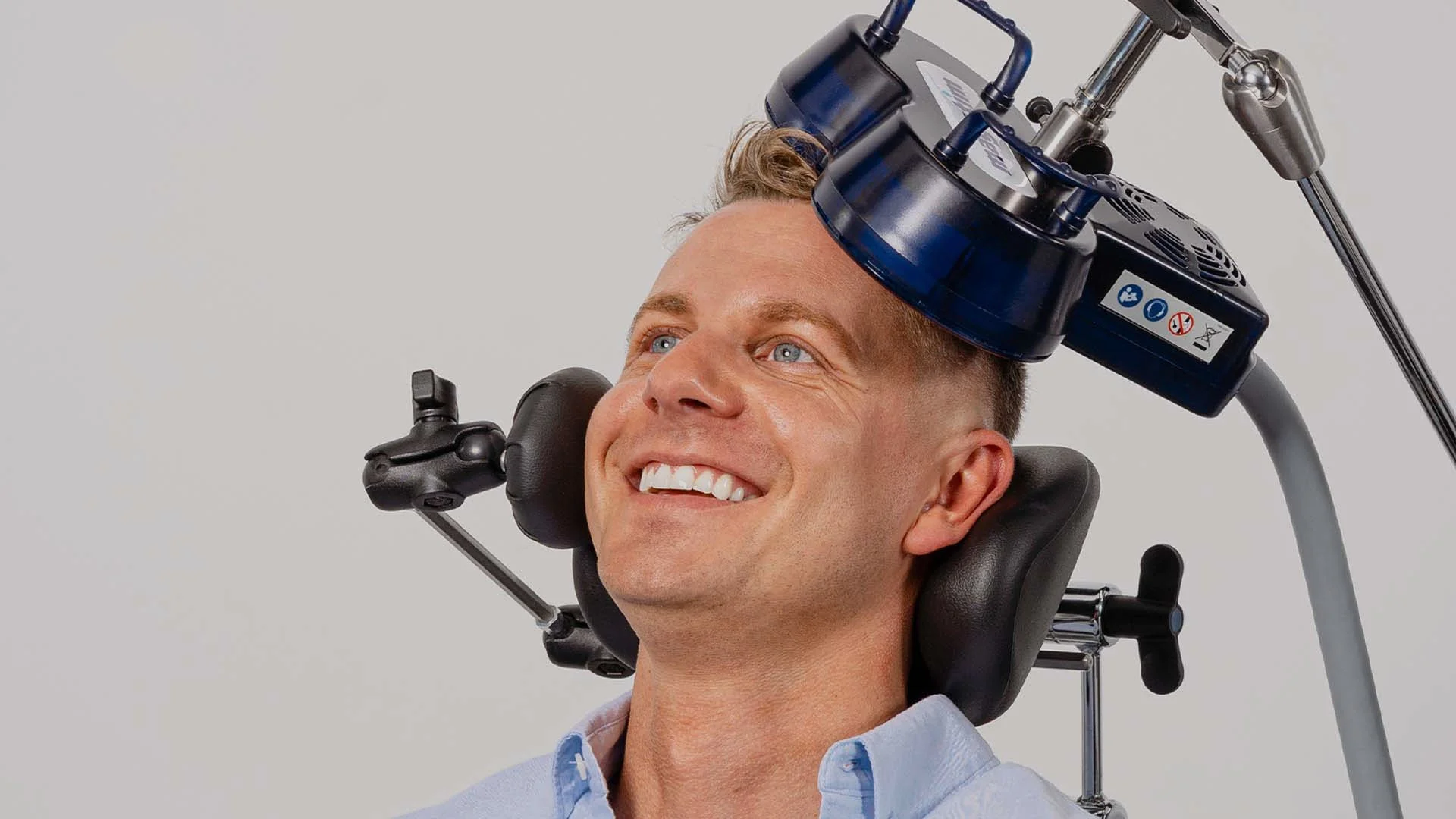
ACCELERATED INTERMITTENT THETA BURST STIMULATION
Southeast Virginia Psychiatry (SeVA) is excited to offer a cutting edge treatment for depression called aiTBS, which stands for accelerated intermittent Theta Burst Stimulation. The term “accelerated” is used to describe any TMS therapy protocol applying more than one TMS session a day. Our accelerated TMS therapy protocol is based on the FDA-cleared SAINT protocol, which shortens the duration of treatment from 6 weeks to just 5 days.
What is SAINT Protocol?
A recent breakthrough in accelerated TMS came from Stanford University and was published in the prestigious peer-reviewed American Journal of Psychiatry in April 2020. Stanford Accelerated Intelligent Neuromodulation Therapy for Treatment-Resistant Depression (SAINT-TRD) demonstrated remarkable efficacy in the treatment of TRD. In this clinical trial, 21 participants with TRD received 10 iTBS sessions per day over 5 consecutive days. Amazingly, 19 of 21 participants (90.48%) met the criteria for remission by the end of the 5th day. Neuropsychological testing demonstrated no negative cognitive side effects; accelerated iTBS was well tolerated and safe. The initial Stanford study has since been supported by further clinical trials, reinforcing the belief in SAINT TMS’s effectiveness.
SeVA’s Accelerated TMS Program
Southeast Virginia Psychiatry offers non-MRI navigated accelerated intermittent theta burst stimulation to select patients. The lack of MRI targeting is the main difference between SeVA Psychiatry’s aiTBS and SAINT TMS. While SAINT TMS protocol is FDA-cleared, SeVA’s aiTBS is not. Unfortunately, machines with MRI navigation systems are not readily accessible to most outpatient TMS clinics, making administration of “true” SAINT TMS difficult to provide outside of a research-based hospital setting. However, there are other methods beyond MRI navigation for identifying the target treatment area, and it hasn’t been demonstrated that MRI navigation is essential in achieving the treatment responses seen in the Stanford trial. At SeVA we utilize an evidence based method called BEAM F3 to identify the target treatment area. Although SeVA’s aiTMS is not FDA-cleared, it is an available, effective, and affordable alternative to SAINT TMS, which may cost up to $20,000.

What to Expect
10 iTBS treatment sessions per day, each lasting 10 minutes and separated by a 50 minute “rest” interval
5 consecutive days of treatment (Monday thru Friday)
Up to 90% chance of seeing remission from depression by the end of the 5th treatment day, with up to 70% likelihood of remaining in remission 1 month later
Because our protocol is not FDA-cleared, insurance will not cover aiTBS treatment
Out of pocket expense that is a fraction of the cost of SAINT TMS, with financing options available